Clinical validation drives digital health safety
There has been an explosion in what many refer to as digital health tech tools, driven by the impact of COVID and the need to make healthcare more readily accessible. However, there is a distinction to be made between telehealth (where human contact is part of the solution) and standalone digital tools. We spoke with our Clinical Product Manager, Polly Sword, to understand more about what clinical validation means and why it is essential, particularly in the development of standalone digital health tech tools.
What is clinical validation and why is it important?
Clinical validation refers to the process of evaluating the safety and effectiveness of a medical device, diagnostic tool, or treatment in a clinical setting. This process involves conducting studies or trials on human subjects to determine whether the product performs as intended and achieves the desired clinical outcomes. In the context of health tech products, clinical validation is crucial for several reasons.
First, it provides evidence that the product is safe and effective for use in real-world clinical settings. This helps to build trust among employers, private medical insurance companies, healthcare providers and patients, who need assurance that the product will not cause harm or lead to incorrect assessment or treatment decisions.
Second, clinical validation can help health tech companies to secure regulatory approval for their products. In many countries, medical devices and diagnostic tools must undergo rigorous testing and evaluation before they can be sold or marketed. Clinical validation provides the data and evidence needed to satisfy regulatory requirements and obtain necessary approvals.
Put simply, clinical validation demonstrates the safety and effectiveness of products through rigorous clinical testing and helps build a strong reputation for quality and reliability.
Why is it even more important for standalone digital tools?
Results, outputs and outcomes of clinically validated standalone tools don’t need to be independently reviewed, approved or released by a clinician. They are shown to be robust and reliable as part of a clinical validation process specific to that product, its intended use and desired effect. And as such, this can increase the accessibility to and flexibility of healthcare provision. There are no time or availability restrictions. Just results.
Looking at MSK specifically, the costs and waiting lists associated with Musculoskeletal problems have long been a problem for healthcare services and it is well accepted that early intervention for MSK issues facilitates and improves recovery, preventing chronicity and associated problems. As such, providing access to a standalone digital tool delivering MSK self-assessment makes perfect sense.
Once clinically validated is a digital tool always validated?
A digital tool only remains validated in its original format and within the original parameters of its intended use. Any changes to a product will compromise its validation. It is therefore important to have a monitoring, review and/or continuous validation process to ensure clinical validation for the lifetime of a product.
How did PhysioWizard achieve clinical validation?
The depth of the clinical background, and the robustness of PhysioWizard® is such that it took a number of years to design, test and clinically validate the tool. The platform was built by physiotherapists, with a combined professional experience of over 100 years, and tested within the NHS using real-world data and is continuously reviewed and tested in live patient environments.
As a result, the current clinically validated PhysioWizard® digital tool, offers an alternative to face-to-face MSK triage that can be quickly and easily accessed by the wider population including those currently restricted in accessing care by physical, social, economic or geographical problems. While as a self-referral service, the self-management outcome enables users to access information for the management of their problem 24/7, reducing the need for other medical services.
There has been an explosion in what many refer to as digital health tech tools, driven by the impact of COVID and the need to make healthcare more readily accessible. However, there is a distinction to be made between telehealth (where human contact is part of the solution) and standalone digital tools. We spoke with our Clinical Product Manager, Polly Sword, to understand more about what clinical validation means and why it is essential, particularly in the development of standalone digital health tech tools.
What is clinical validation and why is it important?
Clinical validation refers to the process of evaluating the safety and effectiveness of a medical device, diagnostic tool, or treatment in a clinical setting. This process involves conducting studies or trials on human subjects to determine whether the product performs as intended and achieves the desired clinical outcomes. In the context of health tech products, clinical validation is crucial for several reasons.
First, it provides evidence that the product is safe and effective for use in real-world clinical settings. This helps to build trust among employers, private medical insurance companies, healthcare providers and patients, who need assurance that the product will not cause harm or lead to incorrect assessment or treatment decisions.
Second, clinical validation can help health tech companies to secure regulatory approval for their products. In many countries, medical devices and diagnostic tools must undergo rigorous testing and evaluation before they can be sold or marketed. Clinical validation provides the data and evidence needed to satisfy regulatory requirements and obtain necessary approvals.
Put simply, clinical validation demonstrates the safety and effectiveness of products through rigorous clinical testing and helps build a strong reputation for quality and reliability.
Why is it even more important for standalone digital tools?
Results, outputs and outcomes of clinically validated standalone tools don’t need to be independently reviewed, approved or released by a clinician. They are shown to be robust and reliable as part of a clinical validation process specific to that product, its intended use and desired effect. And as such, this can increase the accessibility to and flexibility of healthcare provision. There are no time or availability restrictions. Just results.
Looking at MSK specifically, the costs and waiting lists associated with Musculoskeletal problems have long been a problem for healthcare services and it is well accepted that early intervention for MSK issues facilitates and improves recovery, preventing chronicity and associated problems. As such, providing access to a standalone digital tool delivering MSK self-assessment makes perfect sense.
Once clinically validated is a digital tool always validated?
A digital tool only remains validated in its original format and within the original parameters of its intended use. Any changes to a product will compromise its validation. It is therefore important to have a monitoring, review and/or continuous validation process to ensure clinical validation for the lifetime of a product.
How did PhysioWizard achieve clinical validation?
The depth of the clinical background, and the robustness of PhysioWizard® is such that it took a number of years to design, test and clinically validate the tool. The platform was built by physiotherapists, with a combined professional experience of over 100 years, and tested within the NHS using real-world data and is continuously reviewed and tested in live patient environments.
As a result, the current clinically validated PhysioWizard® digital tool, offers an alternative to face-to-face MSK triage that can be quickly and easily accessed by the wider population including those currently restricted in accessing care by physical, social, economic or geographical problems. While as a self-referral service, the self-management outcome enables users to access information for the management of their problem 24/7, reducing the need for other medical services.
Polly Sword has over 30 years’ experience as a physiotherapist in the NHS (junior to management level), private practice (both as a consultant and practice owner) and has spent the last 8 years in digital health (development and implementation). The depth of her experience in physiotherapy and the provision of healthcare has been invaluable in the development of PhysioWizard® and the platform achieving clinical validation.
PhysioWizard®
PhysioWizard®
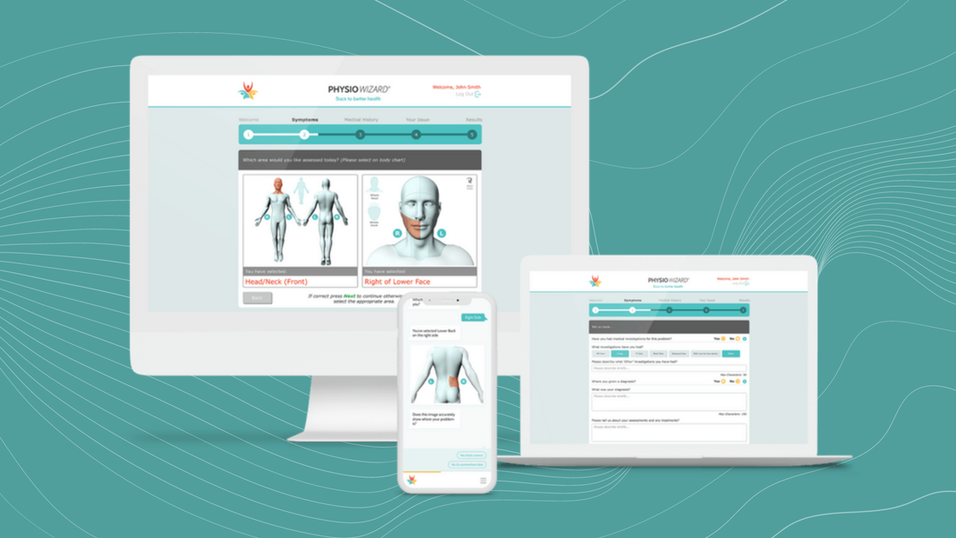
PhysioWizard® is a digital MSK self-assessment tool used by employers, health providers and private medical insurers to give employees, patients and members quick and easy access to an online assessment of 108 body areas. An instant report provides advice, guidance and self-help exercises (where appropriate) then signposts to next stage healthcare treatment (early treatment leads to quicker recovery and prevents chronic pain from developing in those experiencing muscle and joint problems). Built using Augmented Intelligence (AI), PhysioWizard® is the first clinically trialled and validated tool in the UK which intelligently interacts with users to assess with accuracy in a robust and safe way. Available 24/7, it puts better MSK healthcare in your hands.